Abstract
Acquired chronic neutropenia (ACN) in adults is uncommon and its diagnosis can only be asserted after exclusion of primary causes. Once its intrinsic nature is affirmed, the pathophysiologic evaluation of the underlying mechanisms of neutropenia can be challenging because of multiple possible etiologies related to a variety of classical associations.
The diagnosis and the management of patients with ACN may be difficult due to lack of prospective studies and clinical trials. We assembled and analyzed an institutional cohort (2002-2022; n=262) of adult patients (Table 1) with ACN (median age 56 yrs; IQR 43-76) with the goal of reviewing diagnostic and clinical criteria along with molecular NGS-based data, for the inclusion into the clinical consideration of new molecular disease entities, chiefly clonal hematopoiesis (CH).
The patients were categorized based on the severity of neutropenia, anti-neutrophil antibody positivity, presence of splenomegaly, T-cell receptor (TCR) analysis, flow cytometry etc. After exclusion of all extrinsic causes of neutropenia and congenital cases, we found that ACN was associated with T-LGL (37%), autoimmune neutropenia (AIN; 28%), MGUS (6%) and hypogammaglobulinemia (5%). In 84 (35%) patients, the underlying cause of neutropenia was not defined and hence referred to as chronic idiopathic neutropenia (CIN). There was an overlap between some of the clinical associations e.g., T-LGL/AIN coexistence (2%), MGUS (2% AIN and 12% of T-LGL patients). Among CIN patients, 2 seemingly distinct subtypes could be discerned; i) T-cell clonopathy of undetermined significance (TCUS) and ii) CH.
A high T-LGL count along with other potential features of T-LGL (e.g., TCR clonality, despite not fulfilling the diagnostic criteria for T-LGL) was present in 21% of CIN patients, suggesting a diagnostic continuum. Unlike T-LGL, none of these patients had a somatic STAT3 mutation and in 50% of the cases, TCR rearrangement was not diagnostic. We concluded that these CIN cases might be considered as a less polarized version of T-LGL induced neutropenia and therefore designated these patients as having TCUS. Per definition, TCUS showed less pronounced features of T-LGL including the presence/number of T-LGL cells, TCR rearrangement positivity, oligoclonal Vβ utilization pattern and Vβ repertoire skewing.
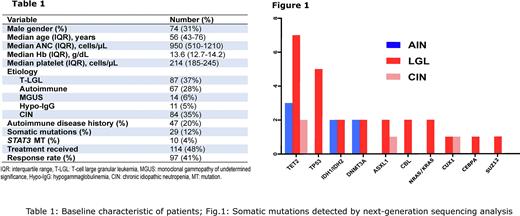
Using the now ubiquitous NGS, CH was identified in 12% of patients with ACN, including 4%, 24%, and 2% of patients with AIN, T-cell associated disease, and CIN, respectively. In addition, CH was observed among AIN/T-LGL and B-cell/T-LGL mediated neutropenia (which accounted for 17% and 3% of all ACN patients with CH). The most common CH was due to TET2 (n=6 patients), TP53 (n=4), IDH1/IDH2 (n=2), DNMT3A (n=2), ASXL1 (n=2), CBL (n=2), NRAS/KRAS (n=2) mutations (Fig.1).
The combined sequential overall response rate (ORR) to pharmacologic therapy was achieved in 85% of all patients. We observed a transient, but high ORR (87%) to growth factors across the board. The ORR to immunosuppressive therapy (IST) i.e., corticosteroids, methotrexate, cyclosporine (CsA), cyclophosphamide, tacrolimus, sirolimus, ATG, and mycophenolate was 43% (45% in T-LGL and 50% in TCUS). One of two patients having T-cell associated disease with co-occuring CH showed absolute neutrophil count improvement with corticosteroids. Notably, neutropenic patients with CH showed a good responsiveness to CsA [6/12 (50%)]; however, no response to either tacrolimus or methotrexate was observed.
Our data show that a significant fraction of ACN appears to be often related to cytotoxic T-cell mediated processes as a part of a continuum starting from TCUS, and may eventually culminate in an overt, fully blown T-LGL. We also detected the presence of CH in a fraction of ACN patients with variable degrees of overlap in others. IST directed against T-cells is a rational treatment choice in ACN with features compatible with a T-cell process and CH, while pure CH with a large clone would warrant workup for MDS. In terms of etiology, we will demonstrate the conundrum of assigning etiology of neutropenia to TCUS/LGL, CH/CCUS vs. other causes at the ASH meeting.
Disclosures
Patel:Alexion: Speakers Bureau; Apellis: Speakers Bureau; Novartis: Speakers Bureau. Carraway:Takeda: Other: DSMB; Novartis: Honoraria, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Speakers Bureau; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Syndax: Other: DSMB; AbbVie: Other: DSMB; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Speakers Bureau. Maciejewski:Apellis Pharmaceuticals: Consultancy; Alexion: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal